Programs
Any information collected on paper is suitable for ScanForm.
ScanForm allows people to continue to write data on paper, as they already do, and uses AI to instantly extract the data with an inexpensive smartphone.
The digitized data then integrates with existing government systems (DHIS2, EMRs, etc.), for a solution that is not siloed.
-
ScanForm has already been deployed across the HIV testing, treatment, and viral load cascade, at community and facility-level in multiple PEPFAR-supported countries in Sub-Saharan Africa.
-
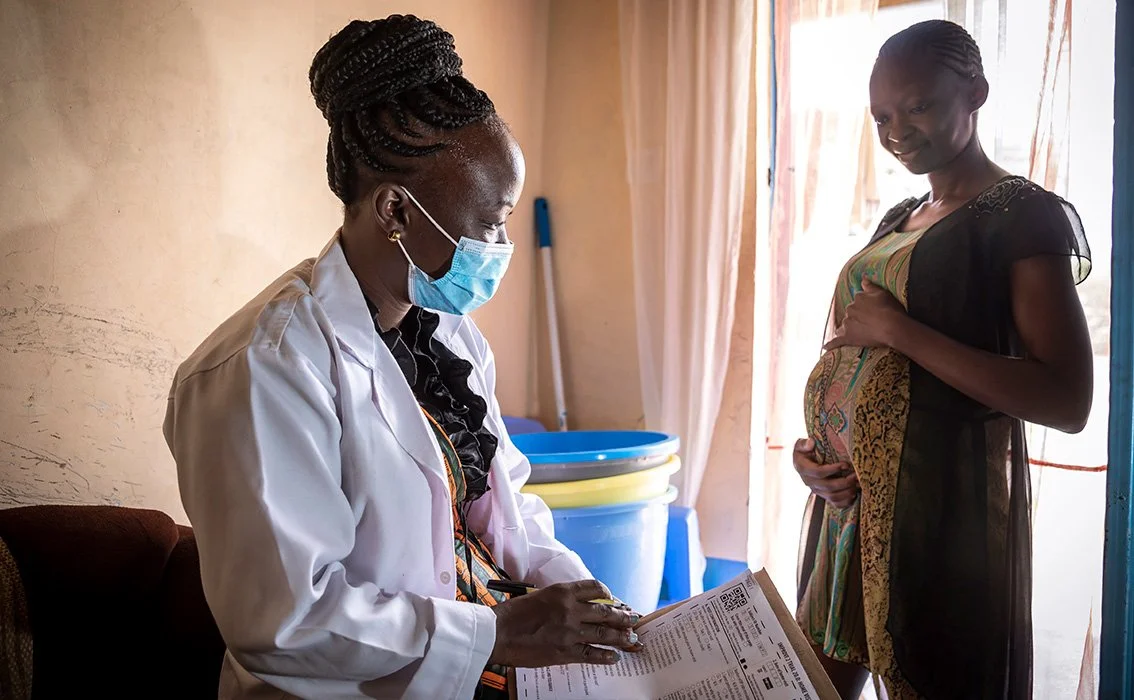
ScanForm has been successfully used for ANC and EMTCT.
In Western Kenya, a scannable patient-carried Maternal Child Handbook was piloted, which longitudinally tracks a pregnancy and the first five years of the child’s life, and contains the equivalent of a dozen routine health registers spanning malaria, HIV, nutrition, and immunization. This pragmatic approach has the potential to yield 5-10 fold cost savings in pharmacovigilance research efforts when used at scale to link pregnancy exposure data (e.g. diseases, drugs, vaccines) to neonatal, infant, and child health.
-
Tracking commodities is essential for accountability of donor investments, and to avoid stock outs of key medications at sites. Current systems still do not meet needs.
One of the critical reasons is that current systems track supply but not consumption. ScanForm, given that it works at every site, automatically tracks consumption of HIV test kits, ART, or whatever supply is being used, by site, in near real-time. It is currently deployed for CAB-LA in Malawi, where it carefully tracks each dose. Because it tracks consumption, it automatically knows when supply is running low and can prompt alerts for re-supply. Additionally, this consumption data, when paired with supply-side data that actually captures records on paper, provides clear data on where supplies are, and where they are being lost.
-
The cornerstone of epidemic detection and control is for an epidemic threat to be detected and reported rapidly so that action can be taken. Current systems in every country leave massive gaps of sites and areas that are uncovered, because the systems deployed don’t work where infrastructure is poor. This gap means that epidemic threats can go unreported for a long time, as happened in West Africa in 2014, leading to billions of dollars in cost and catastrophic loss of life.
ScanForm works everywhere, leaving no gaps. Reporting is in near real-time. It also has automated AI-driven aberration detection, which means that even if a disease occurs only a small amount more commonly across a large number of sites (for example, a 20% increase in diarrhea at 30 sites), the AI can detect that anomaly, even when humans may miss it.
-
ScanForm has been used to support multiple clinical trials in Kenya, Benin, Burkina Faso and Niger. In regions where RCTs were executed, more accurate denominators were captured from scannable routine MOH tools and registers for maternal and child health (eg. antenatal care, postnatal care, MCH handbook, child welfare clinic), outpatient department and community health volunteers.
Status quo:
Studies introduce a parallel one-off electronic system to select sites just for the duration of the clinical trial, introducing whiplash to already overburdened healthcare workers who don't want more brand new temporary systems to deal with.
By repeatedly selecting the same electronically sophisticated facilities, studies unintentionally bias participation toward large urban centers and away from typical service settings.
Unique value proposition:
Innovating routine systems for research: by digitizing every element at the point of capture, ScanForm strengthens routine tools for research-grade data capture. MOH registers already have extensive reach across the country, beyond select trial sites. To capture additional study-specific data, auxiliary tools can be paired with the routine tools.
Representative participation: using ScanForm allows anywhere in the country to be a potential treatment group regardless of infrastructure, with the whole country as the control group.
Baseline data: scannable routine health tools allow the capture of critical baseline information, including demographic health surveillance akin to the DHS.
Measurement of long-term outcomes: by strengthening routine MOH tools with ScanForm, the effects of any clinical trial intervention can still be measured many years afterwards.
Time and cost savings: ScanForm delivers a unique, end‑to‑end data platform that automates analyses, enforces real‐time trial tracking, and embeds continuous data‐quality checks—eliminating cumbersome administrative workflows that accelerate study enrollment and outcome reporting. These efficiencies translate into significant savings and more strategic resource allocation.
A common, paper‐first yet digitally harmonized approach ensures consistency of data collection and provides the MOH with actionable, standardized data to inform policy decisions and program improvements.
The PI of the IMPROVE-2 trial reported saving 9 months of time with ScanForm.
Using a scannable MCH handbook for pharmacovigilance can yield a 5-10x reduction in cost savings, compared to trying to stitch this data together across 12 different registers.
-
ScanForm has been used for school examinations, scholarship applications and student attendance tracking.
In Northern Nigeria, UNICEF used ScanForm to facilitate the rapid retrospective processing of millions of primary school attendance records from the 2021–2022 academic year in Sokoto, Kebbi, Zamfara, and Katsina States. As of June 2022, more than 5,500,000 distinct student attendances and over 386,000 student absences had been tabulated.
During 2023-2024, ScanForm ultimately captured more than 130,000,000 prospective student attendance records from 973 schools.
National Scale
Malawi | Full HIV Testing and Treatment Cascade, STI, OPD, and Supply Chain
2022 - ongoing
After a successful pilot in 2021, ScanForm was rolled out nationally with the 3-test HIV diagnostic algorithm to more than 920 HIV Testing Services (HTS) sites and covering more than 2,300 facility and community access points. MOH-compliant summaries and DHIS2 reports are automatically generated for each site and updated daily, eliminating at least 25% of the manual reporting burden for providers each month.
ScanForm scales rapidly: in less than 6 months, more than 75% of the national HIV testing burden was captured and over 95% coverage was achieved in 1.5 years.
ScanForm captures the full HTS cascade including testing, prevention (PrEP / CAB-LA), ART treatment, and viral load suppression. Over 20 million individual testing records have been digitized, with more health programs continuing to expand to ScanForm including STI, OPD, and stock cards used in the pharmacy.
100%
57 / 57 HTS providers in Malawi reported data collection is faster with ScanForm
- Focus group feedback, CHAI 2021






Cameroon | Community Health and Outreach, HIV Testing Services and additional health programs
2024 - ongoing
Led by the MOH, the Pediatric HIV Surge in Cameroon used ScanForm across 1,000 facilities in all 10 districts. Over 160,000 children and parents in the community were tested for HIV and assessed for TB, hepatitis B, malnutrition, vaccinations, and legal status.
Approximately 3,500 individuals were newly tested positive for HIV and initiated on ART, including over 1,000 children. The surge nearly doubled HIV case finding for children and adolescents, linking clients to care and treatment above the expected 95% target. Now renamed to Find Every Child (FECh) and ongoing, the expansion of ScanForm is continuing.
Working in close collaboration with MOH and ICT, ScanForm is being integrated into the National Digital Health Strategy for multiple programs, including local hosting in the national data center and integration with DHIS2. In 2026, ScanForm is being rolled out nationally with the 3-test HIV diagnostic algorithm with ongoing scoping to additional health programs.
“We were impressed. ScanForm was able to accurately capture diverse handwritings in both English and French and seamlessly digitize them.
The technology enabled teams to save time on data entry and instead use that time to go out and find more children living with HIV that have been missed through routine facility-based services.”
- Dr. Mohamed F. Jalloh, PhD
CDC Cameroon Country Director
Nigeria | HIV Testing Services and PMTCT
2024 - ongoing
In 3 Northwestern and North-Central states, ScanForm is capturing patient-level data for all routine health registers used for HTS and PMTCT. This data is integrated with DHIS2 and LAMIS+, a locally developed and offline EMR system with servers hosted within clinics. Medical staff within the clinic administer the full cycle of data, including writing on paper, digitization with ScanForm, verification of data, clearing of DQAs, and integration of clean data into LAMIS+.
In 2026, ScanForm will expand to 5 CDC-supported states and integrate with NMRS, an OpenMRS-based EMR.









Côte d'Ivoire | HIV Testing Services and additional health programs
2025 - ongoing
ScanForm is currently deployed to 12 facilities to support the new 3-test algorithm for HIV, with further scale up planned for 2026.
Scoping exercises are also ongoing to expand ScanForm to additional health programs including other HIV program tools (ART, laboratory, and PrEP), TB registers, consultation and laboratory tools, pharmacy records, antenatal and neonatal care registers, immunization tools, and documentation for reportable diseases such as cholera, polio, and yellow fever.
Sub-National Scale
-
2024 - 2025
Azithromycine pour la Vie des Enfants au Niger (“Azithromycin [to save] young lives in Niger”) - AVENIR
In collaboration with the University of California San Francisco, ScanForm was piloted in 6 CSIs to collect eligibility and treatment data for children under 5 years of age to receive azithromycin during the second round of MDA of AVENIR.
Community health workers were evaluated and 100% (15/15) preferred ScanForm to the original manual spreadsheet.
-
2023 - 2025
ScanForm versions of the community testing register and index testing register were deployed by Health System Trust to capture HIV community testing data from 80 mobile buses in KwaZulu-Natal Province, South Africa.
-
2023 - ongoing
ScanForm is used by the MOH to support the Influenza Sentinel Surveillance Program in two hospitals, including a Respiratory Syncytial Virus (RSV) surveillance pilot for capturing and analyzing Influenza-Like Illnesses (ILI), Severe Acute Respiratory Illness (SARI) and extended SARI data.
-
2022 - 2024
With authorization from the Ministry of Health and Social Services, a ScanForm pilot for COVID-19 vaccination was carried out in 5 clinics in Khomas region. ScanForm data was integrated with DHIS2 including near real-time dashboards and was hosted on local servers to ensure full in-country data sovereignty. The pilot was well received by healthcare workers, reduced data entry backlogs, and demonstrated potential savings of nearly N$900,000 per year in transcription costs for COVID-19 alone. Parallel scoping across HTS, PMTCT, DAPP, and cervical cancer programs confirmed similar efficiency gains.
-
2022
ScanForm improved malaria control by converting French paper-based records from the long lasting insecticide-treated nets (LLIN) ANC register into real-time digitized data, with custom summary statistics and visual analytics.
A study by MCD Global Health reported that ScanForm allowed researchers to collect 30% more malaria data during antenatal care visits than tablet-based data entry.
-
2020 - 2022
As part of the FIKIA “To Reach” project, QED worked with ICAP mobile hospitals to bring HIV care to rural communities in 3 regions that otherwise lacked easy access. All medical workflows in these trucks, including triage, screening, clinical work, and counseling, were supported by both tablet and desktop-based software from QED, designed with strong consideration for intermittent internet conditions and cybersecurity needs. QED also worked with local experts to optimize the truck routing, and supplied software to bajajis supporting auxiliary ART delivery.
-
2020 - 2022
MalCOV (malaria-covid) is a clinical trial led by LSHTM and LSTM that studied the relationship between malaria and antimalarial drugs on COVID-19, in Burkina Faso and Kenya. ScanForm collected all study data and disaggregated participants into screening, COVID-19 and nested malaria treatment cohorts to understand if malaria infection or treatment affects the clinical course of COVID-19.
-
2018 - 2025
ScanForm has been deployed, at one time or another, across 401 health facilities and 1,592 community units in Western Kenya.
ScanForm was used by 20,000 CHVs carrying out under 5 mortality surveillance in 8 counties to evaluate the RTS,S malaria vaccine.
In Migori County, ScanForm was used in 66 facilities for HIV Case Based Surveillance and linked with KenyaEMR patient records for deduplication in the national data warehouse.
ScanForm was used for multiple health registers in facilities across the counties of Migori and Homa Bay, as part of a USAID-funded effort named Tupime Kaunti ("Check the County") to improve health data quality. After one year, 84% of CHMT officers voted for ScanForm as a superior solution for routine data collection and timely malaria surveillance.
Multiple clinical trials evaluating attractive targeted sugar bait (ATSB), spatial repellents, malaria, pregnancy and maternal and child health outcomes:
Evaluating ATSBs for Malaria Reduction in Kenya, Liverpool School of Tropical Medicine (ClinicalTrials.gov ID: NCT05219565)
Spatial Repellents for the Prevention of Malaria in Kenya, University of Notre Dame (ClinicalTrials.gov ID: NCT04766879)
Improving PRegnancy Outcomes With PReVEntive Therapy in Africa-2, Liverpool School of Tropical Medicine (ClinicalTrials.gov ID: NCT04158713)
Awards & Recognition
AWS Health Equity Initiative 2023 - Winner
For “reducing inequities in care by increasing access to services, reducing disparities by addressing social determinants of health, and leveraging data to promote equitable and inclusive systems of care.”
WHO 2022 Guidelines Featuring ScanForm
ScanForm-based tools for HIV testing in Malawi are featured in the WHO’s guidelines for strategic information and strengthening routine data for impact, as a best practice for countries to adopt.
One of 6 winners amongst 317 international competitors, for “unconventional methods to measure the improvement of primary health care performance in low- and middle-income countries.”